INSUBCONTINENT EXCLUSIVE:
Researchers at the prestigiousSalkInstituteare reporting that they have managed to map the molecular structure of a CRISPR enzyme that could
allow scientists to more precisely manipulate functions within cells.
Over the past several years, CRISPR-Cas9 has seized the public
imagination for its ability to edit genetic code in a way that may correct defects inside individual cells — potentially healing mutations
and preventing the advent of many illnesses.
Specifically, Cas9 enzymes act sort of like scissors, snipping away pieces of genetic code and
swapping them out with a replacement
But these enzymes target DNA, which is the fundamental building block for the development of an organism, and there are growing concerns
that using the enzyme to essentially reprogram the DNA of a cell may cause more harm than good.
As this report inScientific American
illustrates:
Researchpublished on Monday suggests that only the tip of a Titanic-sized iceberg: CRISPR-Cas9 can cause significantly greater
genetic havoc than experts thought, the study concludes, perhaps enough to threaten the health of patients who would one
dayreceiveCRISPR-based therapy.
The results come hard on the heels of twostudiesthat identified a related issue: Some CRISPR&d cells might
be missing a key anti-cancer mechanism and therefore be able to initiate tumors.
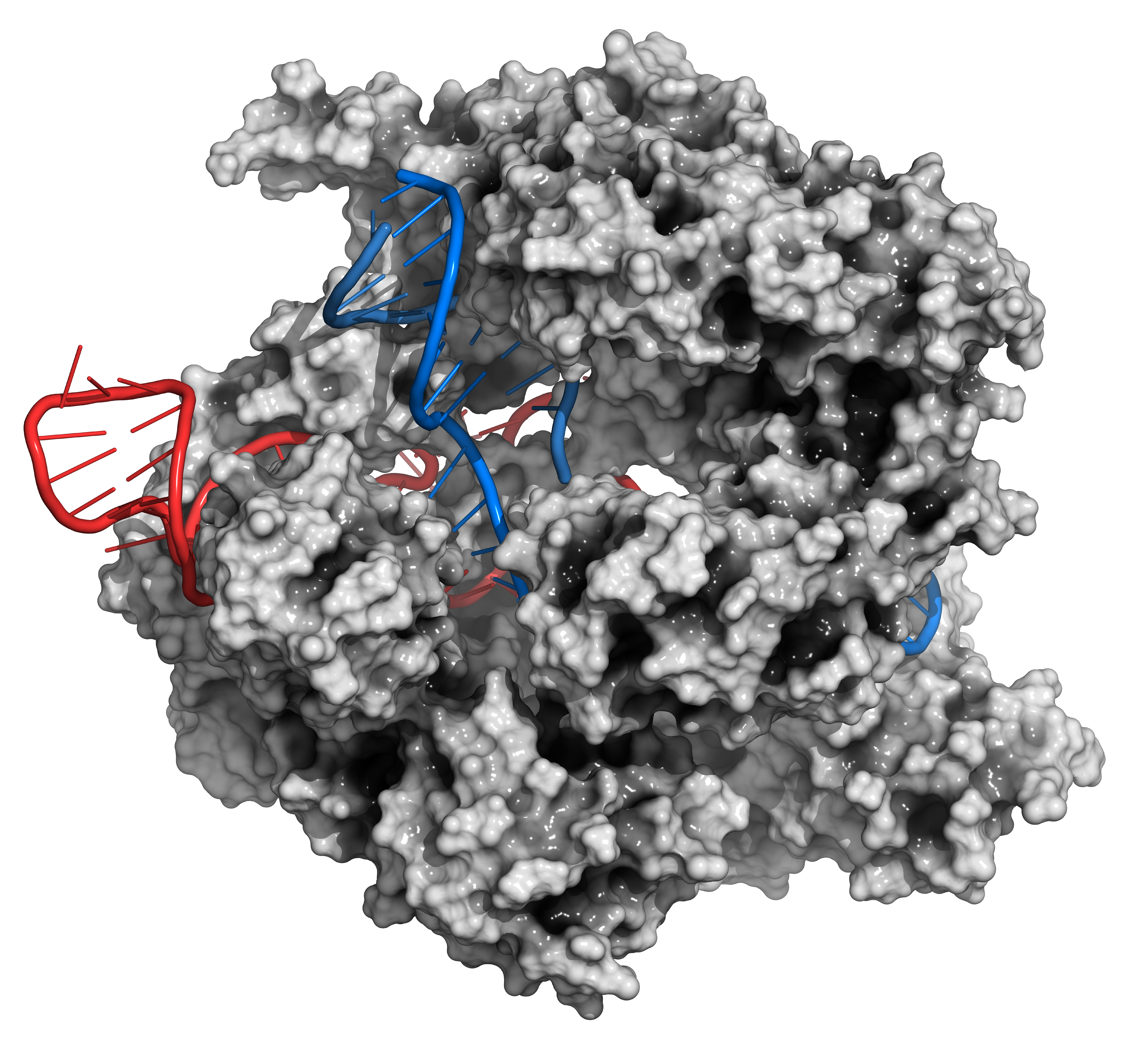
CRISPR-CAS9 gene editing complex from Streptococcus
The Cas9 nuclease protein uses a guide RNA sequence to cut DNA at a complementary site
Cas9 protein: white surface model
DNA fragments: blue ladder cartoon
Photo courtesy Getty Images
The new findings from the Salk Institute, published in the journalCell, provide the detailed molecular structure
of CRISPR-Cas13d, an enzyme that can target RNA instead of DNA.
Once thought to just be the delivery mechanism for instructions encoded in
DNA for cell operations, RNA is now known to carry out biochemical reactions like enzymes, and serve their own regulatory functions in cells
By identifying an enzyme that can target the mechanisms by which cells operate, rather than the overall plan for cellular function,
scientists should be able to come up with even more highly refined treatments with fewer risks.
Put more simply, having editing tools can
allow scientists to modify a gene activity without making permanent — and potentially dangerous — changes to the gene itself seems like
a good option to explore.
DNA is constant, but what always changing are the RNA messages that are copied from the DNA,& saysSalkResearch
Associate Silvana Konermann, a Howard Hughes Medical Institute Hanna Gray Fellow and one of the study first authors, in a statement
&Being able to modulate those messages by directly controlling the RNA has important implications for influencing a cell fate.
Researchers
at Salk first identified the family of enzymes they&re calling CRISPR-Cas13d earlier this year and suggested that this alternate system
could recognize and cut RNA
Their first work was around dementia treatment, and the team showed that the tool could be used to correct protein imbalances in cells of
dementia patients.
In our previous paper, we discovered a new CRISPR family that can be used to engineer RNA directly inside of human
cells,& saidHelmsley-SalkFellow Patrick Hsu, who is the other corresponding author of the new work
&Now that we&ve been able to visualize the structure of Cas13d, we can see in more detail how the enzyme is guided to the RNA and how it is
These insights are allowing us to improve the system and make the process more effective, paving the way for new strategies to treat
RNA-based diseases.
The paper other authors were Nicholas J
Brideau and Peter Lotfy ofSalk; Xuebing Wu of the Whitehead Institute for Biomedical Research; and Scott J
Novick, Timothy Strutzenberg and Patrick R
Griffin of The Scripps Research Institute, according to a statement.